ITCH RESULTS
RAPID AND CLINICALLY MEANINGFUL ITCH RELIEF1-3
KEY SECONDARY ENDPOINT
≥4-POINT IMPROVEMENT IN ITCH (ITCH NRS4*) AT WEEK 81-3
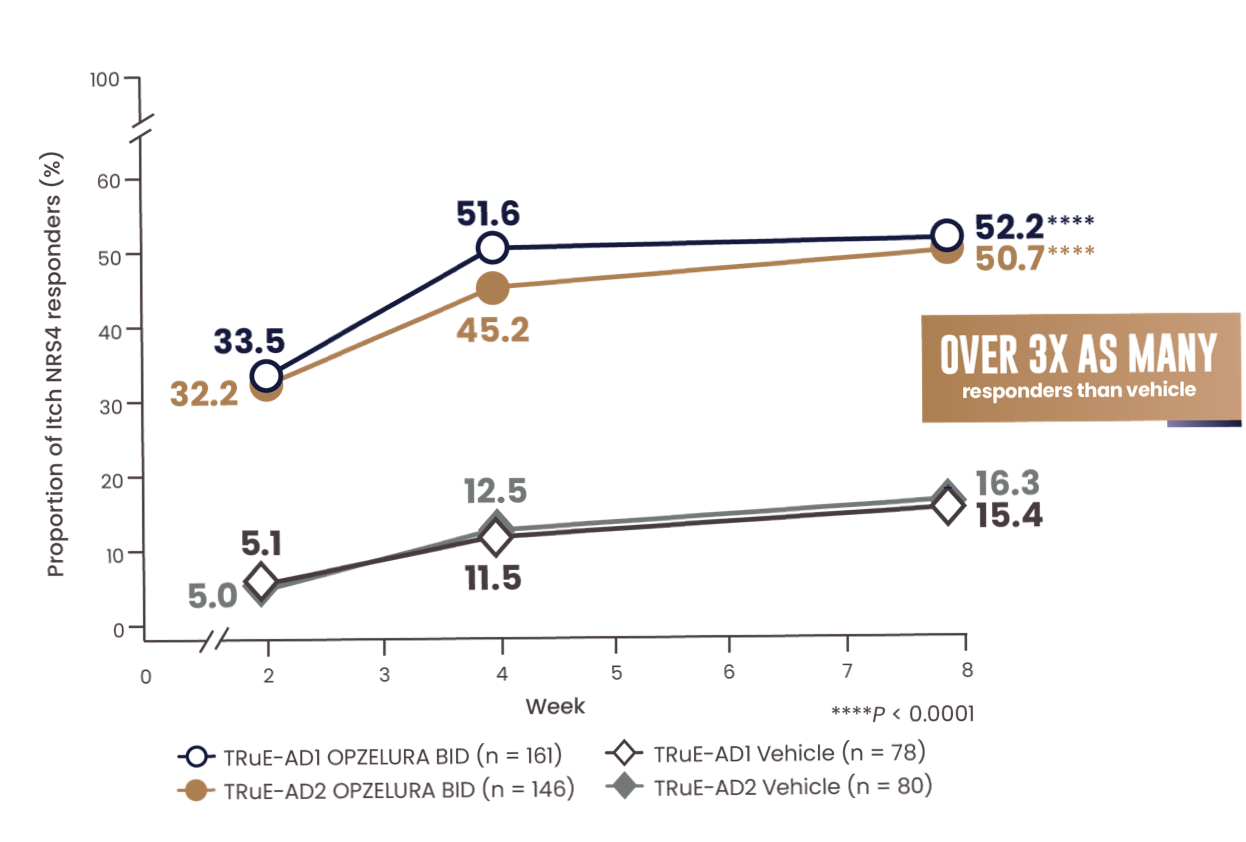
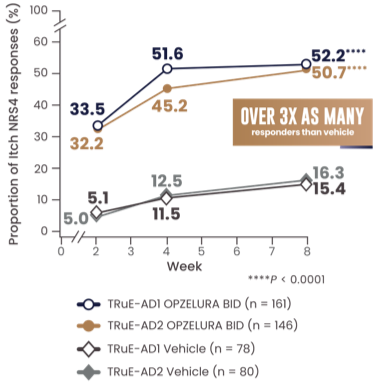
MORE THAN 1 IN 2 PATIENTS ACHIEVED A CLINICALLY MEANINGFUL IMPROVEMENT IN ITCH (NRS4*) AT WEEK 81-3
*Itch NRS4 is defined as the achievement of at least a 4-point improvement in daily itch on a 0- to 10-point scale, considered a clinically meaningful response. Patients in the analysis had an NRS score ≥4 at baseline; mean NRS score at baseline was 5.1,2
BID, twice daily; NRS, numerical rating scale.
Adapted from Papp K et al. doi:10.17632/ffx6nd5zyb.1. Licensed under CC BY 4.0
The line graph above shows the proportion of Itch NRS4 responders for OPZELURA and vehicle from the TRuE-AD1 and TRuE-AD2 clinical trials from Week 0 to Week 8. At Week 8, 52.2% of patients taking OPZELURA achieved a 4-point improvement in itch vs. 15.4% for vehicle in TRuE-AD1. In TRuE-AD2, 50.7% of patients taking OPZELURA achieved a 4-point improvement in itch vs. 16.3% for vehicle. This demonstrates over 3x as many responders to OPZELURA than vehicle.1-3
RAPID IMPACT ON ITCH
>30% of patients achieved Itch NRS4* at Week 2 (33.5% vs. 5.1% and 32.2% vs. 5.0%)2,3
POST-HOC, EXPLORATORY ANALYSIS
Difference in Itch NRS4 was observed as early as
Day 2 (NRS ≥ 4; 11.6% vs. 2.9% and 10.8% vs. 1.3%)4,5
No conclusions around efficacy should be made based on these results.
*Itch NRS4 is defined as the achievement of at least a 4-point improvement in daily itch on a 0- to 10-point scale, considered a clinically meaningful response. Patients in the analysis had an NRS score ≥4 at baseline; mean NRS score at baseline was 5.1,2
NRS, numerical rating scale.
WHAT ARE PATIENTS SAYING ABOUT OPZELURA?
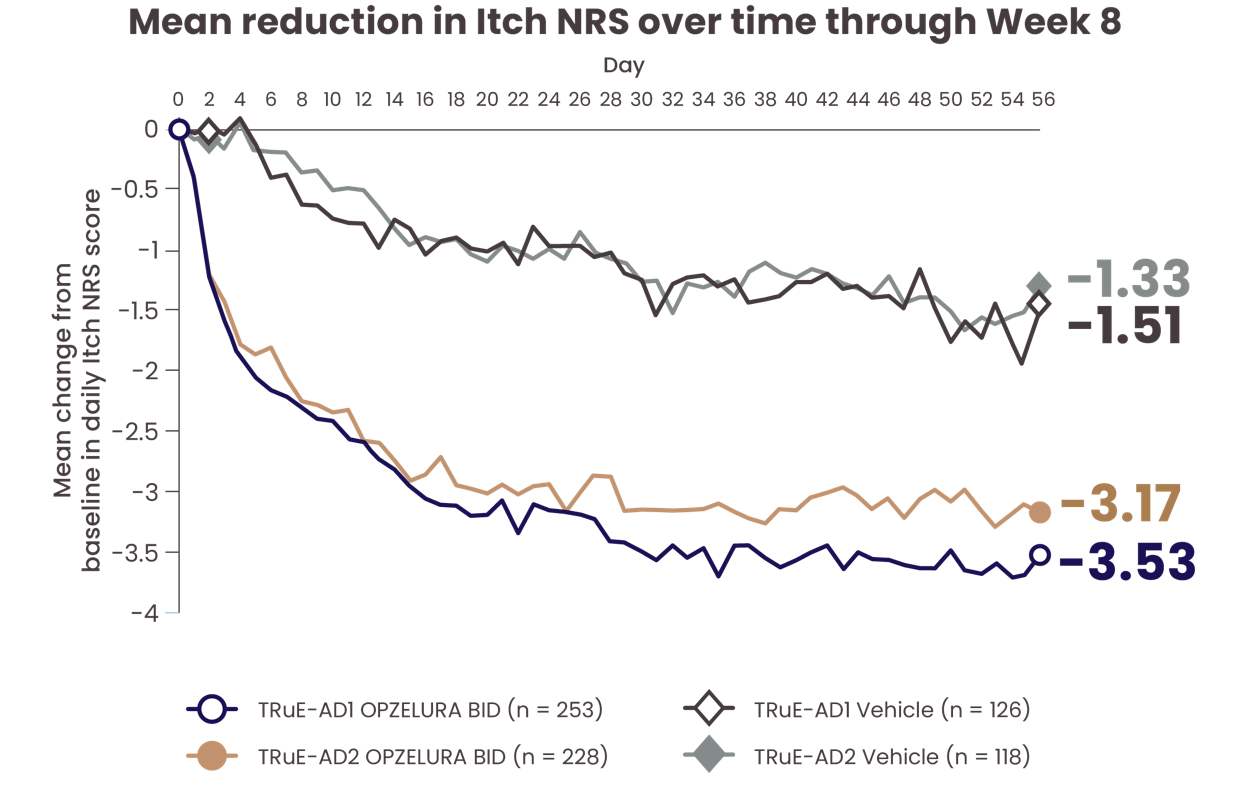
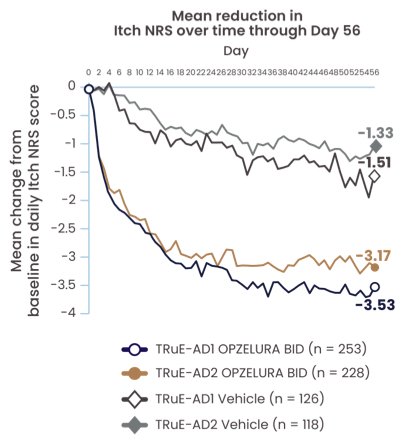
CHANGE IN ITCH NRS* THROUGH WEEK 82
ADDITIONAL EXPLORATORY ANALYSIS
PATIENT-REPORTED CHANGE IN ITCH NRS SCORE OBSERVED AS EARLY AS DAY 1† AND THROUGH DAY 562
Results were not adjusted for multiple comparisons.
Adapted with permission from Papp K et al. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
The line graph above shows the mean reduction in Itch NRS from the TRuE-AD1 and TRuE-AD2 clinical trials from Day 0 to Day 56. In TRuE-AD1 and TRuE-AD2, respectively, patients taking OPZELURA experienced a 3.53% and 3.17% reduction in itch by Day 56 vs. 1.51% and 1.33% for those taking the vehicle.2
*For Itch NRS assessment, patients completed an electronic diary each evening, reporting their worst level of itch during each 24-hour period from 0 (no itch) to 10 (worst imaginable itch).2
†No conclusions of efficacy should be made based on these results.
BID, twice daily; NRS, numerical rating scale.
ITCH-FREE STATE (NRS 0/1*) MEASURED FROM DAY 1 THROUGH DAY 7 –POOLED ANALYSIS6
ADDITIONAL EXPLORATORY ANALYSIS
PATIENTS ACHIEVING ITCH NRS 0/1* IN THE FIRST 7 DAYS6
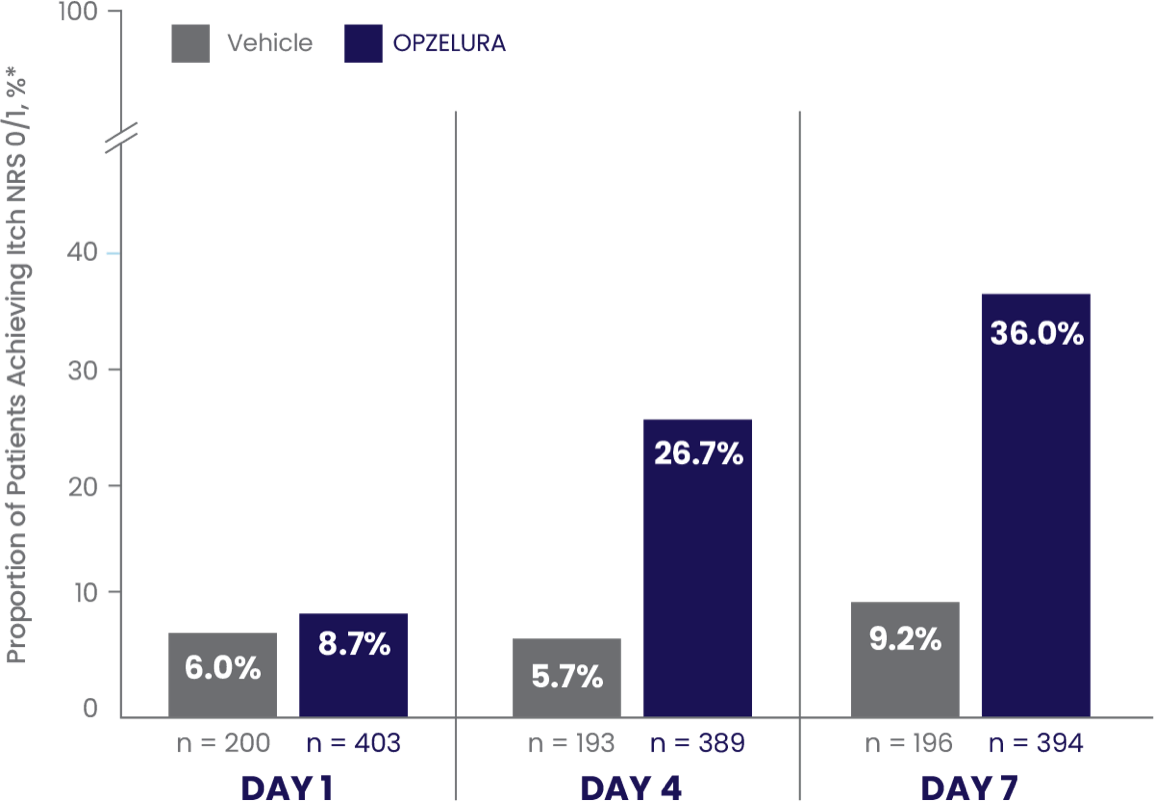
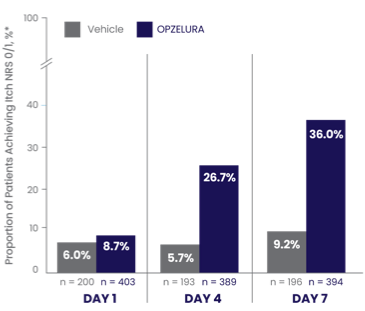
In a pooled analysis the proportion of patients who showed Itch NRS 0/1* at DAY 7 was 36.0% (and 9.2% vehicle)6
- Data were reported as observed
- No conclusions of safety or efficacy should be made based on these results
*Patients in the analysis had an Itch NRS score >1 at baseline.
NRS, numerical rating scale
The bar chart above shows the proportion of clinical trial participants who achieved Itch NRS 0/1 in the first 7 days of treatment. 36% of patients taking OPZELURA showed Itch NRS 0/1 at Day 7 vs. 9.2% for vehicle.6
SCRATCH-AD PHASE 2 STUDY7
An open-label, single-arm study of 46 patients to evaluate the effect of OPZELURA on itch in adult participants with mild to moderate AD.7
SECONDARY ENDPOINT: MEAN (SE) CHANGE FROM
BASELINE IN MPP-NRS SCORE OF 6.45,7
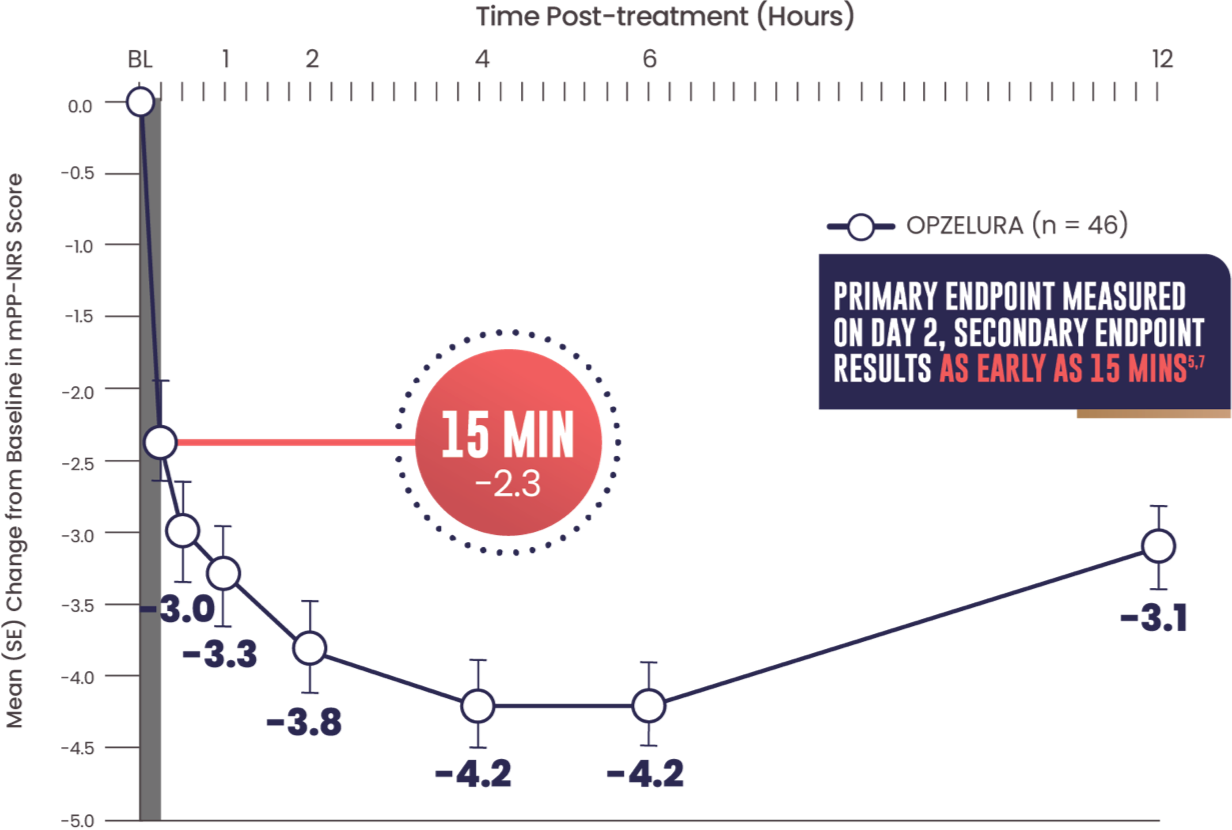
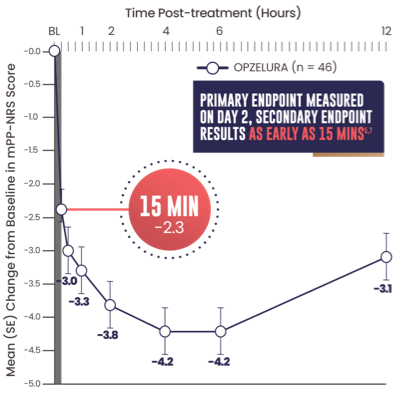
- Primary endpoint: mean change from baseline PP-NRS (6.7) on Day 2 was -3.47
- Secondary endpoint: 15 minutes after the first OPZELURA application the mean change from baseline in mPP-NRS was -2.37
- Data were reported as observed7
- No conclusions of safety or efficacy should be made based on these results
Adapted from Bissonnette et al. Revolutionizing Atopic Dermatitis. 2023.
The line graph above shows that OPZELURA (n = 46) demonstrated a mean change from baseline in mPP-NRS score of -2.3 at 15 minutes. At 30 minutes, the mean change was -3.0. At 1 hour, the mean change was -3.3. At 2 hours, the mean change was -3.8. At 4 and 6 hours, the mean change was -4.2. At 12 hours post treatment, the mean change was -3.1.5,7
Secondary endpoints included: change from baseline in mPP-NRS at 15 and 30 minutes and at 1, 2, 4, 6, and 12 hours post-treatment on Day 1, change from baseline in PP-NRS on Day 3 through Day 29, and change from baseline in IGA at Days 8, 15, and 29.7
TEAEs were reported in 30.6% of participants; all were grade 1 or 2; none were serious.7
AD, atopic dermatitis; BL, baseline; IGA, Investigator’s Global Assessment; min, minutes; mPP-NRS, modified peak pruritus numerical rating scale;
PP-NRS, peak pruritus numerical rating scale (PP-NRS is reported as the worst level of itch during the past 24-hour period from 0 [no itch] to 10 [worst imaginable itch]); SE, standard error; TEAE, treatment-emergent adverse event.


